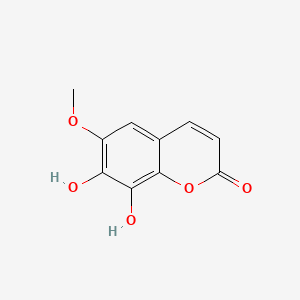
| Phytochemical Name : Fraxetin |
| PCNDIDF0047 |
| Pubchem CID : 5273569 |
| Molecular formula: C10H8O5 |
| Canonical SMILES : COC1=C(C(=C2C(=C1)C=CC(=O)O2)O)O |
Synonymes : Fraxetin|574-84-5|7,8-Dihydroxy-6-methoxycoumarin|7,8-Dihydroxy-6-methoxy-2H-chromen-2-one|7,8-dihydroxy-6-methoxychromen-2-one|2H-1-Benzopyran-2-one, 7,8-dihydroxy-6-methoxy-|UNII-CD3GD44O3K|7,8-Dihydroxy-6-methoxy-2-benzopyrone|CD3GD44O3K|7,8-Dihydroxy-6-methoxy-chromen-2-one|CHEMBL54909|CHEBI:5169|EINECS 209-376-2|7,8-Dihydroxy-6-methoxy-2H-1-benzopyran-2-one|Fraxetol|8-hydroxyscopoletin|Spectrum_001507|SpecPlus_000477|FRAXETIN [MI]|Spectrum2_001639|Spectrum3_001842|Spectrum4_001686|Spectrum5_000332|Oprea1_735469|SCHEMBL43472|BSPBio_003224|Fraxetin, analytical standard|KBioGR_001952|KBioSS_001987|MLS002207123|DivK1c_006573|SPECTRUM1504069|SPBio_001737|MEGxp0_000506|ACon0_001071|ACon1_000442|KBio1_001517|KBio2_001987|KBio2_004555|KBio2_007123|KBio3_002724|DTXSID00205992|7,8-dihydroxy-6-methoxy coumarin|KUC106681N|HY-N0580|TNP00177|Coumarin, 7,8-dihydroxy-6-methoxy|BDBM50206215|CCG-38759|MFCD00006873|s9503|STL564671|Coumarin, 7,8-dihydroxy-6-methoxy-|AKOS000277991|7,8-Dihydroxy-6-methoxycoumarin, 98%|NCGC00017270-01|NCGC00017270-02|NCGC00017270-03|NCGC00017270-04|NCGC00017270-05|NCGC00096046-01|NCGC00096046-02|NCGC00169075-01|NCGC00169075-02|AC-34572|AS-67313|SMR000112323|KSC-11-207-12|CS-0009115|FT-0632418|7,8-Dihydroxy-6-methoxy-2H-chromen-2-one #|A14554|C09265|SR-05000002449|Q-100662|SR-05000002449-1|BRD-K76587808-001-03-8|Q15410973 |
| Structure | |
| 3D structure | 2D structure |
 |
| Log Po/w (iLOGP) : 1.520 |
| Log Po/w (XLOGP3) : 1.170 |
| Log Po/w (WLOGP) : 1.210 |
| Log Po/w (MLOGP) : 0.200 |
| Log Po/w (SILICOS-IT) : 1.450 |
| Consensus Log Po/w : 1.110 |