| Phytochemical Name : Lidocaine |
| PCNDIDL0055 |
| Pubchem CID : 3676 |
| Molecular formula: C14H22N2O |
| Canonical SMILES : CCN(CC)CC(=O)NC1=C(C=CC=C1C)C |
Synonymes : lidocaine|137-58-6|Lignocaine|Xylocaine|2-(Diethylamino)-N-(2,6-dimethylphenyl)acetamide|Lidoderm|Alphacaine|Duncaine|Esracaine|Xylestesin|Cappicaine|Gravocain|Leostesin|Maricaine|Isicaina|Solcain|Xylocain|L-Caine|Isicaine|Xylocitin|Rucaina|Xilina|Xycaine|Cito optadren|Anestacon|Lida-Mantle|Dentipatch|Xylotox|2-(Diethylamino)-2',6'-acetoxylidide|Lidocainum|Lignocainum|Cuivasil|Jetocaine|Octocaine|Remicaine|Xilocaina|Xyloneural (free base)|Dalcaine|2-Diethylamino-N-(2,6-dimethylphenyl)acetamide|Lidocaina|Xllina|ELA-Max|Acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-|ZTlido|Diethylaminoaceto-2,6-xylidide|Lidocaine (VAN)|2',6'-Acetoxylidide, 2-(diethylamino)-|alpha-Diethylamino-2,6-dimethylacetanilide|Xilocaina [Italian]|Xylesthesin|Dilocaine|Lanabiotic|Versatis|Ztilido|Rocephin Kit|Emla Cream|Lidocainum [INN-Latin]|Lidocaina [INN-Spanish]|EMBOLEX|CHEBI:6456|alfa-Dietilamino-2,6-dimetilacetanilide|HSDB 3350|Lidocaine Monohydrochloride|Xylocaine CO2|Dentipatch (TN)|EINECS 205-302-8|Xylocaine (TN)|NSC 40030|NSC-40030|Lidocaine [USAN:INN:JAN]|Lidocaton|Zingo|BRN 2215784|ALGRX 3268|ALGRX-3268|DTXSID1045166|Xylocaine Viscous|UNII-98PI200987|CHEMBL79|LIDOPEN|alfa-Dietilamino-2,6-dimetilacetanilide [Italian]|C14H22N2O|N-(2,6-dimethylphenyl)-N(2),N(2)-diethylglycinamide|Diethylaminoacet-2,6-xylidide|MLS000069724|EMLA COMPONENT LIDOCAINE|98PI200987|DTXCID9025166|ORAQIX COMPONENT LIDOCAINE|SYNERA COMPONENT LIDOCAINE|Lidocaine [USP:INN:BAN:JAN]|FORTACIN COMPONENT LIDOCAINE|LIDOCAINE COMPONENT OF EMLA|4-12-00-02538 (Beilstein Handbook Reference)|NSC40030|LIDOCAINE COMPONENT OF ORAQIX|LIDOCAINE COMPONENT OF SYNERA|2-diethylamino-2',6'-acetoxylidide|LANABIOTIC COMPONENT LIDOCAINE|LIDOCAIN COMPONENT OF FORTACINE|ROCEPHIN KIT COMPONENT LIDOCAINE|.alpha.-Diethylaminoaceto-2,6-xylidide|CDS1_000283|LIDOCAINE COMPONENT OF LANABIOTIC|NCGC00015611-10|SMR000058189|.alpha.-(Diethylamino)-2,6-acetoxylidide|LIDOCAINE COMPONENT OF ROCEPHIN KIT|omega-Diethylamino-2,6-dimethylacetanilide|DIETHYLAMINO-2,6-DIMETHYLACETANILIDE|Lidocaine Base|.alpha.-Diethylamino-2,6-dimethylacetanilide|.omega.-Diethylamino-2,6-dimethylacetanilide|LIDOCAINE (MART.)|LIDOCAINE [MART.]|N-(2,6-Dimethylphenyl)-N2,N2-diethylglycinamide|2-(Diethylamino)-N-(2,6-Dimethylphenyl)ethanamide|2-Diethylamino-N-(2,6-dimethyl-phenyl)-acetamide|Lignocaine hydrochloride|N-(2,6-dimethylphenyl)-N~2~,N~2~-diethylglycinamide|LIDOCAINE (EP MONOGRAPH)|LIDOCAINE [EP MONOGRAPH]|LIDOCAINE (USP MONOGRAPH)|LIDOCAINE [USP MONOGRAPH]|DermaFlex|Anestacon Jelly|Xylocaine-Mpf|Zilactin-L|2-(Diethylamino)-N-(2,6-dimethylphenyl)-acetamide|Lidocaine hydrochloride hydrate|After Burn Gel|Lidoject-1|Lidoject-2|After Burn Spray|Octocaine-50|91484-71-8|CAS-137-58-6|LQZ|Octocaine-100|Xylocaine Test Dose|Xylocaine Endotracheal|Norwood Sunburn Spray|Xylocaine 5% Spinal|2-2EtN-2MePhAcN|Xylocaine Dental Ointment|MFCD00026733|Xylocaine-Mpf with Glucose|Lingocaine|Lidocain|Qualigens|Xyline|Lignocaine base|Lidoderm Patch|After Burn Double Strength Gel|LidocaineHClH2O|After Burn Double Strength Spray|Lidocaine, powder|N1-(2,6-dimethylphenyl)-N2,N2-diethylglycinamide|Zingo (Salt/Mix)|Lidocaine (Alphacaine)|Spectrum_001118|Lidothesin (Salt/Mix)|Xyloneural (Salt/Mix)|LIDOCAINE [INN]|LIDOCAINE [JAN]|Opera_ID_385|LIDOCAINE [MI]|LIDOCAINE [HSDB]|LIDOCAINE [INCI]|Maybridge1_002571|Prestwick0_000050|Prestwick1_000050|Prestwick2_000050|Prestwick3_000050|Spectrum2_001343|Spectrum3_001392|Spectrum4_000070|Spectrum5_001549|LIDOCAINE [VANDF]|Lopac-L-5647|Lidaform HC (Salt/Mix)|D0X4RN|Epitope ID:116205|Lidamantle HC (Salt/Mix)|2', 2-(diethylamino)-|LIDOCAINE [USP-RS]|LIDOCAINE [WHO-DD]|LIDOCAINE [WHO-IP]|Neosporin Plus (Salt/Mix)|Lopac0_000669|SCHEMBL15689|BSPBio_000179|BSPBio_001359|BSPBio_003004|KBioGR_000079|KBioGR_000599|KBioSS_000079|KBioSS_001598|MLS000758263|MLS001074177|MLS001423964|BIDD:GT0342|DivK1c_000174|DivK1c_001323|L1026_SIGMA|Lidocaine, analytical standard|SPBio_001525|SPBio_002100|Lidocaine (JP15/USP/INN)|Lidocaine (JP17/USP/INN)|LIDOCAINE [GREEN BOOK]|BPBio1_000197|GTPL2623|LIDOCAINE [ORANGE BOOK]|SCHEMBL17967359|HMS548M19|KBio1_000174|KBio2_000079|KBio2_001598|KBio2_002647|KBio2_004166|KBio2_005215|KBio2_006734|KBio3_000157|KBio3_000158|KBio3_002224|C01BB01|C05AD01|D04AB01|N01BB02|R02AD02|S01HA07|S02DA01|Lidocaine 1.0 mg/ml in Methanol|LIDOCAINUM [WHO-IP LATIN]|NINDS_000174|Bio1_000379|Bio1_000868|Bio1_001357|Bio2_000079|Bio2_000559|HMS1791D21|HMS1989D21|HMS2051C21|HMS2089E15|HMS2235O14|HMS3371J04|HMS3393C21|HMS3428O07|HMS3651G09|AMY25560|BCP09081|HY-B0185|Tox21_110183|alpha-Diethylaminoaceto-2,6-xylidide|BDBM50017662|LS-805|NSC789222|s1357|STK552033|Solarcaine aloe extraburn relief cream|AKOS001026768|Tox21_110183_1|CCG-100824|CS-2070|CS-O-01810|DB00281|NC00074|NSC-789222|SB19118|SDCCGSBI-0050648.P005|WLN: 2N2 & 1VMR B1 F1|.alpha.-Diethylamino-2,6-acetoxylidide|alpha-(Diethylamino)-2,6-acetoxylidide|CAS-73-78-9|IDI1_000174|IDI1_033829|NCGC00015611-01|NCGC00015611-02|NCGC00015611-03|NCGC00015611-04|NCGC00015611-05|NCGC00015611-06|NCGC00015611-07|NCGC00015611-08|NCGC00015611-09|NCGC00015611-11|NCGC00015611-12|NCGC00015611-13|NCGC00015611-14|NCGC00015611-15|NCGC00015611-16|NCGC00015611-18|NCGC00015611-31|NCGC00022176-05|NCGC00022176-06|NCGC00022176-07|NCGC00022176-08|NCGC00022176-09|AC-10282|AS-13718|SY052029|2-(Diethylamino)-2'',6''-acetoxylidide|SBI-0050648.P004|AB00053581|L0156|SW196598-4|A18187|C07073|D00358|M06299|AB00053581-27|AB00053581-28|AB00053581_29|AB00053581_30|EN300-6472705|LIDOCAINE (73-58-6 (MONOHYDROCHLORIDE)|A833036|Q216935|(2,6-dimethylphenyl)carbamoylmethyl-diethyl-azanium|Acetamida, 2-(dietilamino)-N-(2,6-dimetilfenil)-|N1-(2,6-dimethylphenyl)-2-(diethylamino)acetamide|W-108233|2-(Diethylamino)-N-(2,6-dimethylphenyl)acetamide #|BRD-K52662033-001-02-6|BRD-K52662033-003-05-5|BRD-K52662033-003-14-7|Z55135799|Lidocaine, British Pharmacopoeia (BP) Reference Standard|Lidocaine, European Pharmacopoeia (EP) Reference Standard|N~1~-(2,6-dimethylphenyl)-N~2~,N~2~-diethylglycinamide|Lidocaine, United States Pharmacopeia (USP) Reference Standard|2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide hydrate hydrochloride|Lidocaine, Pharmaceutical Secondary Standard; Certified Reference Material|LIDOCAINE (73-58-6 (MONOHYDROCHLORIDE); 6108-05-0 (MONOHYDROCHLORIDE MONOHYDRATE)) |
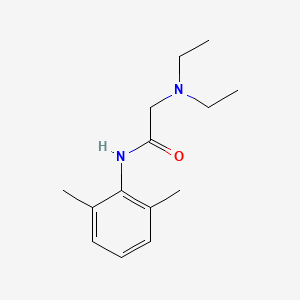
| Structure | |
| 3D structure | 2D structure |
 |
| Log Po/w (iLOGP) : 2.860 |
| Log Po/w (XLOGP3) : 2.260 |
| Log Po/w (WLOGP) : 2.390 |
| Log Po/w (MLOGP) : 2.380 |
| Log Po/w (SILICOS-IT) : 2.640 |
| Consensus Log Po/w : 2.500 |